dr Michał Grzybek (Membrane Biochemistry, Paul Langerhans Institute Dresden, Medical Faculty TU Dresden), Modulacja aktywności Receptora Naskórkowego Czynnika Wzrostu przez lipidy (Modulation of Epidermal Growth Factor Receptor activity by lipids); 22.11.2016, godz. 9:00; sala 1.03
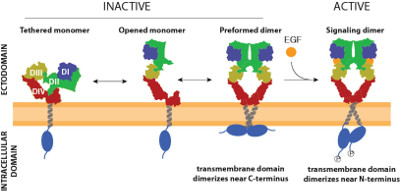
Epidermal growth factor receptor (EGFR) belongs to the receptor tyrosine kinase family (RTK), which play a fundamental role in regulation of cell growth, development and metabolism in multicellular organisms. Its function as a sensitive signaling receptor is intertwined by multiple, not very strong, control mechanisms, which prevent ligand-independent stimulation. The primary layer of signal regulation is ligand binding, which induces structural changes in the receptor’s ectodomain, removes the steric constraints of that domain and allows dimerization and activation of the receptor, initiating the signaling process. Interestingly, EGFR shows two different binding affinities for its prime ligand – EGF, as represented by a concave up Scatchard plot. This dual nature of EGF-binding is thought to be a consequence of the fact that EGFR pool present at the plasma membrane is not homogenous with most of the receptors present as monomers and the rest, present as preformed inactive dimers or even oligomers of higher state. We present a synthetic approach to decipher the interaction of the EGF receptor with various lipids in a proteoliposomal system, in which the lipid composition as well as phase properties of the membrane are controlled. In this minimal system we show that membrane phase heterogeneity is essential to keep the EGF receptor in an autoinhibited state, preventing aberrant activation of the receptor. We also show that only in proteoliposomes with membrane phase heterogeneity the presence of GM3 but not other gangliosides inhibits EGF receptor autophosphorylation by allosteric interaction with the receptor ectodomain, while ligand-binding properties are not affected. Our results demonstrate that GM3 exhibits the potential to regulate the allosteric structural transition from inactive to a signaling EGFR dimer, by preventing the autophosphorylation of the intracellular kinase domain in response to ligand binding. Finally, we show that the high-affinity binding of EGF is regulated by direct interactions between the positively charged surface of the intracellular domain of EGFR and the plasma membrane phosphoinositides.
As other tyrosine receptor kinases such as the human insulin receptor (IR) as well as the vascular endothelial growth factor receptor-2 (VEGFR-2) were also reported to be modulated by lipids, we believe that our proposed regulatory mechanism might be of more general nature. Therefore we hope that our studies will stimulate other scientist with different backgrounds, such as structure biologist, to explore our findings in more detail.